|
Product manufacturers, Chemicals manufacturing Companies, India, exporter,
cas No. 11 Product suppliers, Chemicals, india, companies, Chemicals Drug
from India, Product import, Chemicals, india, Product export, Chemicals,
india, Product buyer, Chemicals, india, Product seller, Chemicals,
india,IP,BP,USP,EP, Product manufacturer, Chemicals, india, Product dealers,
Chemicals, india, Product producer, Chemicals, india, Product, Chemicals,
Chemical ingradients Purchase, Cheap, Lowest, Price, COA,wholesalers,
Chemical india, Product suppliers, Chemicals india, Product trader,
Chemicals, india, Product purchase, Chemicals, india, Product structure,
chemicals, Product benefits, Product pharmacy, drugs, & Chemicals review,
definition of Product, Product synthesis, Product salt, effects of
Product, clinical advantage of Product, polymorphs of Product, preparation
of Product, efficacy of Product, beneficial effects of Product,
determination and validation of Product, determination and validation of
Product, Product -Product, side effect of Product, polymorphs of Product,
level of Product, Product, process for preparing Product, Product mg,
process for Product, detailed info for Product, catalog Product, polymorphs
of Product, dosage, chemicals of Product, intermediate of Product, Product
powder, supply Product, Taj Pharmaceuticals Limited., chemicals
manufacturers India, manufacturers of industrial chemicals, chemicals
suppliers, chemicals offers, chemicals wholesalers, exporters
chemicals, specialty chemicals, chemicals Products, indian chemical
suppliers, chemicals india company, Indian chemical manufacturers
association, chemical industries in India, indian chemical company,
chemical factories india, safety Products manufacturers India, plastic
Products manufacturers india |
|
|
|
|
 |
|
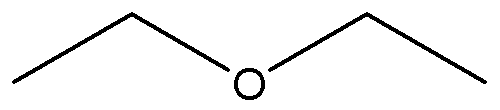
Diethyl ether
CAS number 60-29-7
Identification
Synonyms: Ether; ether,anhydrous; Diethyl ether; 1,1'Oxybisethane;ethyl
oxide; diethyl oxide; Ethyl ether anhydrous;
CAS No.: 60-29-7
Molecular Weight: 74.12
Chemical Formula: C2H5OC2H5
EC No: 200-467-2
Annex I Index No: 603-022-00-4
Physical data
Appearance: colourless liquid
Melting point: -116 C
Boiling point: 34.6 C
Specific gravity: 0.71
Vapour pressure: 400 mm Hg at 18 C
Flash point: -40 C
Explosion limits: 1.7% - 48%
Autoignition temperature: 170 C
Water solubility: 6.9% (20 C)
Stability
Stable, but light-sensitive, sensitive to air. May contain BHT
(2,6-di-tert-butyl-4-methylphenol) as a stabilizer. Substances to be
avoided include zinc, halogens, halogen-halogen compounds, nonmetals,
nonmetallic oxyhalides, strong oxidizing agents, chromyl chloride,
turpentine oils, turps substitutes, nitrates, metallic chlorides.
Extremely flammable. This material is a serious fire and explosion risk.
Vapour may travel considerable distances to ignition sources, which need
not be an open flame, but may be a hot plate, steam pipe, etc. Vapour
may be ignited by the static electricty which can build up when ether is
being poured from one vessel into another. If large quantities of
material are being poured, suitable precautions must be taken.
May form explosive peroxides on storage. Peroxides are generally
higher-boiling than the materials from which they form. Consequently, if
a peroxide-containing solution is heated, the residue becomes
progressively more concentrated in peroxide and the risk of explosion
increases rapidly. Vapour-air mixture explosive (note wide explosion
limits). As at 6.11.97 regulated in the UK under the Highly Flammable
Liquids and Liquefied Petroleum Gases Regulations 1972.
Toxicology
Harmful by ingestion, inhalation or through skin contact. May cause
inebriation or coma. May cause allergic reaction. Skin, eye and
respiratory system irritant. Typical 8h TWA 400 ppm.
Toxicity data
ORL-MAN LDLO 260 mg kg-1
ORL-RAT LD50 1215 mg kg-1
IHL-MUS LC50 31000 ppm/30m Irritation data EYE-HMN 100 ppm
SKN-RBT 360 mg open mld
SKN-GPG 50 mg/24h sev Risk phrases R12 R19 R22 R66 R67.
Personal protection
Remove any source of ignition from the working area, including hot
plates, bunsen burners, hot air guns and electrical equipment.
Only work in very well ventilated areas.
Wear safety glasses.
If using ether from a container that has been open for some time, test
the liquid for the presence of peroxides before use.
Store under an inert atmosphere.
Disposal
Do not attempt to flush this material down a sink. Dangerous levels of
vapour can build up in the sink or within sewers. Store in a waste
solvent container for disposal.
Protective equipment
Safety glasses. Diethyl ether may have a defatting effect on the skin.
If gloves are required, polyvinyl alcohol (PVA) is suggested.
Diethyl ether, also known as ether and ethoxyethane, is a clear,
colorless, and highly flammable liquid with a low boiling point and a
characteristic odor. It is the most common member of a class of chemical
compounds known generically as ethers.
It is an isomer of butanol. Diethyl ether has the formula
CH3-CH2-O-CH2-CH3.
It is used as a common solvent and has been used as a general
anesthetic. Ether is sparingly soluble in water (6.9 g/100 mL). The
diffusion of diethyl ether in air is 0.918*10^-5 m2/s (298K 101.325 kPa).
Diethyl ether is a great solvent for many things, but is extremely
flammable. Professional chemists will be well appraised of the hazards
presented in using ether, but the layperson is less likely to be aware
of these dangers. Diethyl Ether vapors 'hug' the ground, and in dry air
explosive peroxides can form. In other words, even in a spark/flame free
environment, explosions can still happen when ether vapour is
encountered.
For this reason its probably a good idea to have some way of removing
vapours from the vicinity (a fume hood would be a fine example) and (Zaphraud
suggests) one should not use ether on days with extremely low humidity.
Because diethyl ether is so flammable, and prone to ingition, this
procedure should be carried out using a hotplater/stirrer designed for
use in flammable environments. Such a heater/stirrer does not produce a
contact spark when the hotplate is turned on, and generally employs a
brushless AC motor for the stirrer, because DC motors with brushes
generally produce small sparks which could ignite any stray vapours.
Diethyl ether is prepared from ethanol (a.k.a grain alcohol, ethyl
alcohol, drinking alcohol) by heating it with concentrated. The reaction
proceeds thru an intermediary, "Ethyl sulfuric acid", as do most
reactions of this type.
| |
|
Note /Government
Notification: These chemicals are designated as those that are used
in the manufacture of the controlled substances and are important to
the manufacture of the substances. For any (Control Substance)
products Import and Export *** subjected to your country government
laws /control substance ACT.
Information: The information on this web page is provided to help
you to work safely, but it is intended to be an overview of hazards,
not a replacement for a full Material Safety Data Sheet (MSDS). MSDS
forms can be downloaded from the web sites of many chemical
suppliers. ,also that the information on the PTCL Safety web site,
where this page was hosted, has been copied onto many other sites,
often without permission. If you have any doubts about the veracity
of the information that you are viewing, or have any queries, please
check the URL that your web browser displays for this page. If the
URL begins "www.tajapi.com/www/Denatonium Benzoate.htm/" the page is
maintained by the Safety Officer in Physical Chemistry at Oxford
University. If not, this page is a copy made by some other person
and we have no responsibility for it.
The Controlled Substances Act (CSA) was enacted into law by the
Congress of the United States as Title II of the Comprehensive Drug
Abuse Prevention and Control Act of 1970.[1] The CSA is the federal
U.S. drug policy under which the manufacture, importation,
possession, use and distribution of certain substances is regulated.
The Act also served as the national implementing legislation for the
Single Convention on Narcotic Drugs |
|
|
|
|
|








