|
Para
Anisyl Bromide manufacturers, Chemicals manufacturing Companies, India,
exporter, cas No. 104-92-7 Para Anisyl Bromide suppliers, Chemicals, india,
companies, Chemicals Drug from India, Para Anisyl Bromide import, Chemicals,
india, Para Anisyl Bromide export, Chemicals, india, Para Anisyl Bromide
buyer, Chemicals, india, Para Anisyl Bromide seller, Chemicals,
india,IP,BP,USP,EP, Para Anisyl Bromide manufacturer, Chemicals, india, Para
Anisyl Bromide dealers, Chemicals, india, Para Anisyl Bromide producer,
Chemicals, india, Octreotide Acetate, Chemicals, Chemical ingradients
Purchase, Cheap, Lowest, Price, COA,wholesalers, Chemical india, Para Anisyl
Bromide suppliers, Chemicals india, Para Anisyl Bromide trader, Chemicals,
india, Para Anisyl Bromide purchase, Chemicals, india, Para Anisyl Bromide
structure, chemicals, Para Anisyl Bromide benefits, Para Anisyl Bromide
pharmacy, drugs, & Chemicals review, definition of Octreotide Acetate, Para
Anisyl Bromide synthesis, Para Anisyl Bromide salt, effects of Octreotide
Acetate, clinical advantage of Octreotide Acetate, polymorphs of Octreotide
Acetate, preparation of Octreotide Acetate, efficacy of Octreotide Acetate,
beneficial effects of Octreotide Acetate, determination and validation of
Octreotide Acetate, determination and validation of Octreotide Acetate, Para
Anisyl Bromide -Octreotide Acetate, side effect of Octreotide Acetate,
polymorphs of Octreotide Acetate, level of Octreotide Acetate, Octreotide
Acetate, process for preparing Octreotide Acetate, Para Anisyl Bromide mg,
process for Octreotide Acetate, detailed info for Octreotide Acetate,
catalog Octreotide Acetate, polymorphs of Octreotide Acetate, dosage,
chemicals of Octreotide Acetate, intermediate of Octreotide Acetate, Para
Anisyl Bromide powder, supply Octreotide Acetate, Taj Pharmaceuticals
Limited., chemicals manufacturers India, manufacturers of industrial
chemicals, chemicals suppliers, chemicals offers, chemicals
wholesalers, exporters chemicals, specialty chemicals, chemicals
Octreotide Acetates, indian chemical suppliers, chemicals india
company, Indian chemical manufacturers association, chemical industries
in India, indian chemical company, chemical factories india, safety
Octreotide Acetates manufacturers India, plastic Octreotide Acetates
manufacturers india |
|
|
|
|
 |
|
Para Anisyl Bromide
CAS Registry Number: 104-92-7
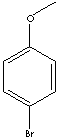
IUPAC Name: 1-bromo-4-methoxybenzene
Synonyms: p-Bromoanisole, Anisyl bromide, p-Bromanisole, Anisole,
p-bromo-, p-Anisyl bromide, 1-Bromo-4-methoxybenzene, 4-BROMOANISOLE,
p-Methoxybromobenzene, 4-Methoxybromobenzene, p-Methoxyphenyl bromide,
4-Methoxyphenyl bromide, Benzene, 1-bromo-4-methoxy-,
4-bromomethoxybenzene, 4-Methoxy-1-bromobenzene, p-Bromophenyl methyl
ether, B56501_ALDRICH, NSC 8042, EINECS 203-252-1, NSC8042, ZINC00404306
Molecular Formula: C7H7BrO
Molecular Weight: 187.033880
PRODUCT IDENTIFICATION
CAS NO 104-92-7
p-BROMOANISOLE
EINECS NO. 203-252-1
FORMULA BrC6H4OCH3
MOL WT. 187.04
H.S. CODEDERIVATION
TOXICITY
Oral, mouse LD50: 2200 mg/kg
SYNONYMS
4-Bromoanisole; 4-Bromo-1-methoxybenzene;
p-Anisyl bromide; 1-Bromo-4-methoxybenzene; p-Bromophenyl methyl ether;
p-Methoxybromobenzene;
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
PHYSICAL STATE
clear to slightly yellow liquid
MELTING POINT 9 - 11 C
BOILING POINT 223 C
SPECIFIC GRAVITY 1.494
SOLUBILITY IN WATER immiscible
pH
VAPOR DENSITY 4.28
AUTOIGNITION NFPA RARING
Health: 1; Flammability: 0; Reactivity: 0
REFRACTIVE INDEX 1.563
FLASH POINT 94 C
APPLICATIONS
Ether is any of a number of organic compounds characterized by an oxygen
atom joined with single bonds by two carbon atoms that are part of
hydrocarbon groups. The general formula is R-O-R', where R and R' are
alkyl or aromatic groups. Ethers are formed by the condensation of two
alcohols by heating with sulfuric acid; the reaction is one of
dehydration. Ethers can be prepared from alkyl halide reacted with
metallic alkoxide (called Williamson synthesis). Ethers are similar to
alcohols but are generally less dense, less soluble in water, and have
lower boiling points. They are relatively unreactive, which makes them
valuable solvents. But ethers will be cleaved at high temperatures by
concentrated hydrogen halides. Ethers have relatively low boiling point
compare to alkanes as they don't form hydrogen bonds each other.
Ethers are more lipophilic than esters [R-C(=O)-O-R']or amides
[RCO-NH2]. Ethers are widely used as solvents for various organic
reactions because they are relatively the least reactive among common
organic compounds except alkanes and fluorocarbons. The common reaction
of ethers is cleavage of the C–O bond by strong acids either in linear
chain or cyclic structure. Ethers in which oxygen is bonded to primary
and secondary alkyl groups can form peroxide compounds in the presence
of gaseous oxygen due to two unpaired electrons in oxygen. Ethers can
act as Lewis bases in chemical reactions. Commonly, ethers are named
simply in listing the alkyl groups in alphabetical order or alkane order
such as ethyl methyl ether or methyl ethyl ether, which is methoxyethane
in IUPAC nomenclature ( the formula of "alkoxyalkane" ).
When ether is a parts of complex molecule or aromatic derivatives, it is
described as an alkoxy substituent such as methoxybenzene ( trivial name
is anisole). The methoxy prefix indicates the function methyl group
joined by single bonds to an oxygen atom, with the general formula
-O-CH3. Cyclic ethers have ring structure where the oxygen has become
part of the ring. The term of epoxide indicate three membered cyclic
ether (also called oxirane) in which an oxygen atom is joined to each of
two carbon atoms that are already bonded to each other; four membered
cyclic ether is called oxetane; five membered cyclic ether, furan (or
oxolane); six membered cyclic ether, pyran (also called oxane)
respectively. Their unhindered oxygen atom carries two unshared pairs of
electrons - a structure which favors the formation of coordination
complexes and the solvation of cations. Cyclic ethers are used as
important solvents, as chemical intermediate and as monomer for
ring-opening polymerization. Crown Ether is a macrocyclic polyether
whose structure contains hydrogen, carbon and oxygen atoms. Each oxygen
atoms are confined between two carbon atoms and exhibits a conformation
with a hole (accordingly called "crown"). Anisole is one of the simplest
aromatic compound to which ether group is linked. But it is different
with aromatic compounds like furan where the oxygen is a part of the
ring.
Anisole, C6H5OCH3 (methyl phenyl ether), is a clear liquid that is
soluble in ether and alcohol; insoluble in water; boiling point 155 C.
Anisole and its derivatives are used as solvents and in perfumery.
Anisole can be obtained from anise seed. Anisic acid, p-methoxybenzoic
acid, is a part of cresol class antiseptic compounds. It is also used as
an insect repellent and ovicide. Anisole, anisic acid, and their
derivatives are also widely used in chemical reaction as intermediates
to obtain target materials such as dyes, pharmaceuticals, perfumes,
photoinitiators and agrochemicals.
| |
|
Note /Government
Notification: These chemicals are designated as those that are used
in the manufacture of the controlled substances and are important to
the manufacture of the substances. For any (Control Substance)
products Import and Export *** subjected to your country government
laws /control substance ACT.
Information: The information on this web page is provided to help
you to work safely, but it is intended to be an overview of hazards,
not a replacement for a full Material Safety Data Sheet (MSDS). MSDS
forms can be downloaded from the web sites of many chemical
suppliers. ,also that the information on the PTCL Safety web site,
where this page was hosted, has been copied onto many other sites,
often without permission. If you have any doubts about the veracity
of the information that you are viewing, or have any queries, please
check the URL that your web browser displays for this page. If the
URL begins "www.tajapi.com/www/Denatonium Benzoate.htm/" the page is
maintained by the Safety Officer in Physical Chemistry at Oxford
University. If not, this page is a copy made by some other person
and we have no responsibility for it.
The Controlled Substances Act (CSA) was enacted into law by the
Congress of the United States as Title II of the Comprehensive Drug
Abuse Prevention and Control Act of 1970.[1] The CSA is the federal
U.S. drug policy under which the manufacture, importation,
possession, use and distribution of certain substances is regulated.
The Act also served as the national implementing legislation for the
Single Convention on Narcotic Drugs |
|
|
|
|
|







