
|
Zolpidem tartrate
|
|
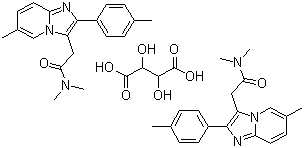
Name Zolpidem tartrate
Synonyms
N,N,6-Trimethyl-2-p-tolyl-imidazo(1,2-a)pyridine-3-acetamide L-(+)-tartrate
Molecular Structure : Zolpidem tartrate,
N,N,6-Trimethyl-2-p-tolyl-imidazo(1,2-a)pyridine-3-acetamide L-(+)-tartrate
(2:1),
CAS No. : : 99294-93-6
Molecular Formula : 2(C19H21N3O).C4H6O6
Molecular Weight : 764.88 |
Zolpidem is a prescription
medication used for the short-term treatment of insomnia, as well as
some brain disorders. It is a short-acting nonbenzodiazepine hypnotic
that potentiates gamma-aminobutyric acid (GABA), an inhibitory
neurotransmitter, by binding to gamma-aminobutyric acid (GABAA)
receptors at the same location as benzodiazepines It works quickly
(usually within 15 minutes) and has a short half-life (2–3 hours).ts
hypnotic effects are similar to those of the benzodiazepine class of
drugs, but it is molecularly distinct from the classical benzodiazepine
molecule and is classified as an imidazopyridine. Flumazenil, a
benzodiazepine receptor antagonist, which is used for benzodiazepine
overdose, can also reverse zolpidem's sedative/hypnotic and memory
impairing effects.As an anticonvulsant and muscle relaxant, the
beneficial effects start to emerge at 10 and 20 times the dose required
for sedation, respectively.[8] For that reason, it has never been
approved for either muscle relaxation or seizure prevention. Such
drastically increased doses are more inclined to induce one or more
negative side-effects, including hallucinations and/or amnesia.
Zolpidem tartrateis a non-benzodiazepine hypnotic of the imidazopyridine
class and is available in 5 mg and 10 mg strength tablets for oral
administration.Zolpidem tartrate is a white to off-white crystalline
powder that is sparingly soluble in water, alcohol, and propylene
glycol.
 INDICATIONS INDICATIONS
zolpidem tartrateis indicated for the short-term treatment of insomnia
characterized by difficulties with sleep initiation.
DOSAGE AND ADMINISTRATION
The doseshould be individualized.
Dosage in adults
The recommended dose for adults is 10 mg once daily immediately before
bedtime. The total Ambien dose should not exceed 10 mg per day.
Special populations
Elderly or debilitated patients may be especially sensitive to the
effects of zolpidem tartrate. Patients with hepatic insufficiency do not
clear the drug as rapidly as normal subjects. The recommended dose of
Ambien in both of these patient populations is 5 mg once daily
immediately before bedtime
Use with CNS depressants
Dosage adjustment may be necessary when Ambien is combined with other
CNS depressant drugs because of the potentially additive effects
Administration
The effectbe slowed by ingestion with or immediately after a meal.
HOW TO USE
Take this medication by mouth, usually once nightly immediately before
bedtime on an empty stomach, or as directed by your doctor. Do not take
it with food because the effect of the medication will be delayed.
Dosage is based on your medical condition, age, and response to therapy.
Do not take more than 10 milligrams per day.
Although unlikely, this drug can infrequently cause temporary memory
loss. To avoid this effect, do not take a dose of this drug unless you
have time for a full night's sleep that lasts at least 7-8 hours. For
example, do not take zolpidem during an overnight plane flight of less
than 8 hours. SIDE EFFECTS: Dizziness, lightheadedness, headache, upset
stomach, diarrhea, and dry mouth may occur. To minimize the risk of
falls, remember to get up slowly when rising from a seated or lying
position. If any of these effects persist or worsen, notify your doctor
or pharmacist promptly.
This medication may make you sleepy during the day. Tell your doctor if
you have daytime drowsiness. Your dose may need to be adjusted.
Rarely, after taking this drug, people have gotten out of bed and driven
vehicles while not fully awake ("sleep-driving"). People have also
sleepwalked, prepared/eaten food, made phone calls, or had sex while not
fully awake. Often, these people do not remember these events. If you
discover that you have experienced any of these events, tell your doctor
immediately. A very serious allergic reaction to this drug is unlikely,
but seek immediate medical attention if it occurs. Symptoms of a serious
allergic reaction may include: rash, itching, swelling (especially of
the face, lips, tongue, or throat), severe dizziness, trouble breathing.
PRECAUTIONS
Before using this medication, tell your doctor or pharmacist your
medical history, especially of: kidney disease, liver disease,
mental/mood problems (e.g., depression), personal or family history of
regular use/abuse of drugs/alcohol/other substances, lung/breathing
problems (e.g., chronic obstructive pulmonary disease-COPD, sleep
apnea), a certain muscle disease (myasthenia gravis).
This drug may make you dizzy or drowsy; use caution engaging in
activities requiring alertness such as driving or using machinery. Avoid
alcoholic beverages because they may increase the risk of this drug's
side effects.
STORAGE
Store at room temperature between 68-77 degrees F (20-25 degrees C) away
from light and moisture. Do not store in the bathroom. Keep all
medicines away from children.
PRECAUTIONS
Abnormal thinking and behavioral changes
A variety of abnormal thinking and behavior changes have been reported
to occur in association with the use of sedative/hypnotics. Some of
these changes may be characterized by decreased inhibition (e.g.,
aggressiveness and extroversion that seemed out of character), similar
to effects produced by alcohol and other CNS depressants. Visual and
auditory hallucinations have been reported as well as behavioral changes
such as bizarre behavior, agitation and depersonalization. In controlled
trials, < 1% of adults with insomnia who received zolpidem reported
hallucinations. In a clinical trial, 7.4% of pediatric patients with
insomnia associated with attentiondeficit/hyperactivity disorder
(ADHD), who received zolpidem reported hallucinations
 Note:
These API/ chemicals are designated as
those that are used in the manufacture of the controlled substances and
are important to the manufacture of the substances. For any (Control
Substance) products Import and Export *** subjected to your country
government laws /control substance ACT. Note:
These API/ chemicals are designated as
those that are used in the manufacture of the controlled substances and
are important to the manufacture of the substances. For any (Control
Substance) products Import and Export *** subjected to your country
government laws /control substance ACT.
Note /Government Notification:
These chemicals are designated as those that are used in the manufacture
of the controlled substances and are important to the manufacture of the
substances. For any (Control Substance) products Import and Export ***
subjected to your country government laws /control substance ACT.
Information: The information on this web page is provided to help you
to work safely, but it is intended to be an overview of hazards, not a
replacement for a full Material Safety Data Sheet (MSDS). MSDS forms can
be downloaded from the web sites of many chemical suppliers. ,also that
the information on the PTCL Safety web site, where this page was hosted,
has been copied onto many other sites, often without permission. If you
have any doubts about the veracity of the information that you are
viewing, or have any queries, please check the URL that your web browser
displays for this page. If the URL begins "www.tajapi.com/www/Denatonium
Benzoate.htm/" the page is maintained by the Safety Officer in Physical
Chemistry at Oxford University. If not, this page is a copy made by some
other person and we have no responsibility for it.
The Controlled Substances Act (CSA) was enacted into law by the Congress
of the United States as Title II of the Comprehensive Drug Abuse
Prevention and Control Act of 1970.[1] The CSA is the federal U.S. drug
policy under which the manufacture, importation, possession, use and
distribution of certain substances is regulated. The Act also served as
the national implementing legislation for the Single Convention on
Narcotic Drugs.

|
|





 INDICATIONS
INDICATIONS




