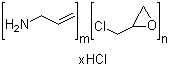|

Name: Sevelamer hydrochloride
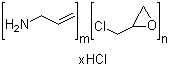
CAS Number : 152751-57-0
Synonyms: 2-(chloromethyl)oxirane; prop-2-en-1-amine; hydrochloride;
2-Propen-1-amine,hydrochloride,polymer with (chloromethyl)oxirane;
RenaGel (TN); Sevelamer hydrochloride (JAN/USAN);Sevelamer HCL; RenaGel;
Molecular Formula: (C3H7N)m. (C3H5ClO)n.(HCl)x
Assay: 99% min
An Improved process for preparation of Sevelamer hydrochloride having
phosphate binding capacity of 4.7 to 6.4 mmol/g comprising the steps of:
(a) dissolving polyallylamine hydrochloride in water to obtain an
aqueous solution; (b) partially neutralizing the aqueous solution of
polyallylamine hydrochloride with 65 to 70 mole % of alkali with respect
to polyallylamine hydrochloride; (c) charging dispersing agent to
hydrocarbon solvent to obtain solution; (d) mixing partially neutralized
aqueous polyallylamine hydrochloride solution with the solution obtained
in step (c); (e) stirring the obtained reaction mixture at speed of
about 40 to about 250 revolutions per minute to get fine dispersion of
aqueous phase in organic phase; (f) heating the suspension obtained in
step (e) at elevated temperature; (g) charging 5 to 12 % by weight of
epichlorohydrin with respect to polyallylamine hydrochloride to the
suspension of step (f) maintaining elevated temperature till cross
linking is complete; (h) cooling the reaction mixture at temperature of
25 to 35° C. and isolating the compound by washing the obtained cake
with water and (i) drying the wet cake in a Fluidized Bed Dryer at
temperature of about 25° C. to about 90° C. to get Sevelamer
hydrochloride with phosphate binding capacity of 4.7 to 6.4 mmol/g.
Detailed offer Description
Sevelamer Hydrochloride is a polymeric amine that binds phosphate and is
meant for oral administration. Sevelamer hydrochloride is
poly(allylamine hydrochloride) crosslinked with epichlorohydrin in which
forty percent of the amines are protonated. It is known chemically as
poly (allylamine-co-N,N'-diallyl- 1,3-diamino-2-hydroxypropane)
hydrochloride. Sevelamer hydrochloride is hydrophilic, but insoluble in
water.
Object Of The Invention
The main object of the present invention is to provide industrial
process for preparation of Sevelamer hydrochloride having PA in the
range of about 4.7 mmol/g to about 6.4 mmol/g and chloride content in
the range of about 3.74 to about 5.60 meq/g.
Another object of the invention is to provide pharmaceutical
compositions comprising a therapeutically effective amount of Sevelamer
hydrochloride along with suitable pharmaceutically acceptable excipients.
Another object of the invention is to provide a novel process for
preparation of Sevelamer hydrochloride compositions comprising high
shear non-aqueous granulation.
Another object of the invention is to provide improved and simplified
process for preparation of Sevelamer hydrochloride which will eliminate
the use of acetonitrile and the risk of gelling also avoid use of IPA
for removing water.
Another object of the invention is to provide Sevelamer hydrochloride
which will meet the stringent ICH (International Committee of
Harmonisation) requirements.
Yet another object of the invention is to provide process which yields
Sevelamer hydrochloride having consistency in degree of cross linking
and avoids the need of specialized equipments for the manufacture of the
said product and thereby reducing the manufacturing cost.
Still another object of the invention is to provide compositions for the
control of serum phosphorus in patients with Chronic Kidney Disease
(CKD) on hemodialysis.
Another object of the invention is to provide method for reducing the
serum phosphorus in patients with Chronic Kidney Disease (CKD) on
hemodialysis comprising administering a therapeutically effective amount
of Sevelamer hydrochloride along with suitable pharmaceutically
acceptable excipients.
Detailed Description Of The Invention
The present invention describes an industrial process for the
preparation of Sevelamer hydrochloride. The present invention further
involves improved process for crosslinking polyallylamine hydrochloride
dispersed in an organic medium with epichlorohydrin to obtain Sevelamer
hydrochloride having phosphate binding capacity of 4.7 to 6.4 mmol/g.
The present invention further describes pharmaceutical compositions
comprising a therapeutically effective amount of Sevelamer hydrochloride
along with suitable pharmaceutically acceptable excipients. A novel
process for preparation of said Sevelamer hydrochloride compositions
comprising high shear non-aqueous granulation is also described.
According to one embodiment of the invention process for preparation of
Sevelamer hydrochloride according to the invention comprises the steps
of;
(a) dissolving polyallylamine hydrochloride in water to obtain an
aqueous solution;
(b) partially neutralizing the aqueous solution of polyallylamine
hydrochloride with 65 to 70 mole % of alkali with respect to
polyallylamine hydrochloride;
(c) charging dispersing agent to hydrocarbon solvent to obtain solution;
(d) mixing partially neutralized aqueous polyallylamine hydrochloride
solution with the solution obtained in step (c);
(e) stirring the obtained reaction mixture at speed of about 40 to about
250 revolutions per minute to get fine dispersion of aqueous phase in
organic phase;
(f) heating the suspension obtained in step (e) at an elevated
temperature;
(g) charging 5 to 12% by weight of epichlorohydrin with respect to
polyallylamine hydrochloride to the suspension of step (f) maintaining
an elevated temperature till cross linking is complete;
(h) cooling the reaction mixture at temperature of 25 to 35° C. and
isolating the compound by washing the obtained cake with water and
filtration;
(i) drying the wet cake in a Fluidized Bed Dryer at temperature of about
25 to 90° C. to get Sevelamer hydrochloride with phosphate binding
capacity of 4.7 to 6.4 mmol/gm.
Sevelamer hydrochloride prepared by the process described by the present
invention is used in formulating Sevelamer hydrochloride compositions.
Phosphate binding polymer Sevelamer is water insoluble but it swells in
contact with water. Due to this tendency of swelling, formulating
Sevelamer by aqueous granulation becomes difficult. Although attempts
have been made to formulate Sevelamer by wet granulation method, none of
the prior art discloses a successful process for high shear non-aqueous
granulation being carried out in an equipment such as a high shear rapid
mixer granulator or a planetary mixer.
Inventors of the present invention attempted granulation of Sevelamer
hydrochloride using spray granulation technique. However, the results
were not satisfactory since the binding solution containing
ethylcellulose dissolved in isopropyl alcohol was very viscous and posed
problem for uniform spraying of the granulating fluid on to the active
ingredient and also the dry mass becomes tacky and forms sticky lumps.
Attempts were also made for preparation of Sevelamer hydrochloride
compositions by hot melt granulation and hot melt extrusion techniques
but the results were not satisfactory as very high quantity of binder
was required and granules produced were lacking adequate flow
properties.
Although the prior art states that tableting of a phosphate binding
polymer such as Sevelamer hydrochloride is impossible by wet
granulation, the inventors of the present invention have successfully
developed a novel process for granulation of Sevelamer hydrochloride by
high shear non-aqueous granulation.
According to the present invention, the process for preparation of
Sevelamer hydrochloride compositions comprising high shear non-aqueous
granulation comprises the steps of:
(a) preparing a mixture of Sevelamer hydrochloride and one or more
diluents;
(b) optionally wetting the prepared mixture;
(c) preparing a non-aqueous binder solution by dissolving binder in an
organic solvent;
(d) granulating the mixture of step (a) or step (b) with non-aqueous
binder solution by high shear non-aqueous granulation to form granulated
mass;
(e) drying the granulated mass;
(f) milling the dried mass using ball mill or fluid energy mill to form
granules of suitable size;
(g) lubricating the milled granules;
(h) compressing the lubricated granules into tablets or filling the
lubricated granules into capsules;
(i) coating the compressed tablets.
According to the invention, the particles of Sevelamer hydrochloride are
round in shape, particularly spherical or oval in shape. Spherical or
oval shaped particles of Sevelamer hydrochloride have low bulk density
and poor flowability and further resist size reduction. Particles resist
deformation and do not rupture or fracture. Due to these characteristics
of Sevelamer hydrochloride, formulating Sevelamer hydrochloride by
direct compression method becomes extremely difficult. In the practice
of the present invention, although the spherical morphology and
hydrophilic nature of active ingredient Sevelamer hydrochloride presents
a special challenge to the formulator, the inventors of the present
invention have successfully prepared Sevelamer hydrochloride
compositions by high shear non-aqueous granulation and by using rapid
mixer granulator or planetary mixer.
Sevelamer hydrochloride is not a free flowing powder and is bulky.
Wetting with purified water helps in decreasing the interparticulate
distance and increasing the contact area between the particles; thus
making Sevelamer Hydrochloride more amenable for the non-aqueous
granulation. Wetting is carried out either in a rapid mixer granulator
or a planetary mixer. In the practice of the present invention, wetting
of mixture of active and diluent is carried out using about 2% to 9% by
weight of purified water. Alternatively, the mixture of active and
diluent may be made wet using a solution of polyethylene glycol
dissolved in purified water. In an alternate method, polyethylene glycol
6000 may be added into the dry mix as a fine powder during the mixing
step. Polyethylene glycols of various grades may be used such as
polyethylene glycol 6000 or the like.



PDF DOWNLOAD
WORD DOCUMENT
Details
|
|