
|
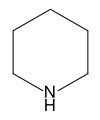
Piperidine and its salts
|
|
Identifiers
CAS number : 110-89-4
Molecular formula : C5H11N
Molecular weight : 85.15 g/mol
Appearance : colourless liquid
Density : 0.862 g/ml, liquid
Melting point : −7 °C
Boiling point : 106 °C
Solubility in water : miscible
Acidity : (pKa) 11.24
Viscosity : 1.573 cP at 25 °C
Hazards : EU classification Flammable (F)
R-phrases : R11, R23/24, R34 |
Piperidine is an organic compound with
the molecular formula (CH2)5NH. This heterocyclic amine consists of a
six-membered ring containing five methylene units and one nitrogen atom.
It is a colorless fuming liquid with an odor described as ammoniacal,
pepper-likehe name comes from the genus name Piper, which is the Latin
word for pepper.Piperidine is a widely used building block and chemical
reagent in the synthesis of organic compounds, including
pharmaceuticals.
Stability
Stable. Highly flammable. Incompatible with strong oxidizing agents,
strong acids, organic acids, water. Vapours may flow along surfaces to a
distant source of ignition.
Toxicology
Poison. May be fatal if inhaled or swallowed. Severe irritant. Skin
contact may cause severe irritation or burns. Contact with the eyes may
lead to permanent damage.
Toxicity data
ORL-RAT LD50 400 mg kg-1
IPR-MUS LD50 50 mg kg-1
SKN-RBT LD50 320 mg kg-1
ORL-MUS LD50 30 mg kg-1
ORL-RBT LD50 145 mg kg-1
Risk phrases
R11 R23 R24 R34. (Note: The risk phrases here are those given by Annex
I. Annex I does not specify R22 or R25, but this material is clearly
harmful if swallowed so should be treated as though R22 were included.]
Personal protection
Safety glasses, gloves, good ventilation. Remove sources of ignition
from the working area.
Piperidine is an organic compound with the molecular formula (CH2)5NH.
This heterocyclic amine consists of a six-membered ring containing five
methylene units and one nitrogen atom. It is a colorless fuming liquid
with an odor described as ammoniacal, pepper-likethe name comes from the
genus name Piper, which is the Latin word for pepper. Piperidine is a
widely used building block and chemical reagent in the synthesis of
organic compounds, including pharmaceuticals.
a colorless, liquid hydrocarbon, (CH)NH, found in many alkaloids and
obtained by reducing pyridine or by treating piperine with alkali: used
in making rubber, oils, fuels, etc.
Production
Industrially, piperidine is produced by the hydrogenation of pyridine,
usually over a molybdenum sulfide catalyst.
Uses
Piperidine is used as a solvent and as a base. The same is true for
certain derivatives: N-formylpiperidine is a polar aprotic solvent with
better hydrocarbon solubility than other amide solvents, and
2,2,6,6-tetramethylpiperidine is highly sterically hindered base, useful
because of its low nucleophilicity and high solubility in organic
solvents.
Reactions
Piperidine is a widely used secondary amine. It is widely used to
convert ketones to enamines
Enamines derived from piperidine can be used in the Stork enamine
alkylation reaction.Piperidine can be converted to the chloramine
C5H10NCl with calcium hypochlorite. The resulting chloramine undergoes
dehydrohalogenation to afford the cyclic imine.
GENERAL DESCRIPTION & APPLICATIONS
Piperidine, hexahydropyridine, is a family of heterocyclic organic
compound derived from pyridine through hydrogenation. It has one
nitrogen atom in the cycle. It is a clear liquid with pepper-like aroma.
It boils at 106 C, soluble in water, alcohol, and ether. The major
application of piperidine is for the production of dipiperidinyl dithium
tetrasulfide used as a rubber vulcanization accelerator. In
pharmaceutical synthesis industry, it is a skeleton in some drugs such
as methylphenidate (central nervous system stimulant), budipine (antiparkinsonian
drug) raloxifene (selective estrogen receptor modulator), minoxidil (an
oral drug to treat high blood pressure). It is used as a special solvent
in solid phase synthesis and a protecting group for peptide synthesis.
Piperidine derivative compounds are used as intermediate to make crystal
derivative of aromatic nitrogen compounds containing nuclear halogen
atoms. Ring system compounds with nitrogen which have basic property
playing important roles as cyclic component in industrial field such as
raw materials for hardener of epoxy resins, corrosion inhibitors,
insecticides, accelerators for rubber, urethane catalysts, antioxidants
and as a catalyst for silicone esters. They are used in manufacturing
pharmaceuticals. Piperidine is listed as a Table II precursor under the
United Nations Convention Against Illicit Traffic in Narcotic Drugs and
Psychotropic Substances.
SPECIFICATION
APPEARANCE : clear to light yellow liquid
PURITY (G.C) : 99.5% min
COLOR, APHA : 10 max
SPECIFIC GRAVITY: 0.857 - 0.867
MOISTURE : (K.F) 0.1% max
PACKING : 170kgs in drum
 Note:
These API/ chemicals are designated as
those that are used in the manufacture of the controlled substances and
are important to the manufacture of the substances. For any (Control
Substance) products Import and Export *** subjected to your country
government laws /control substance ACT. Note:
These API/ chemicals are designated as
those that are used in the manufacture of the controlled substances and
are important to the manufacture of the substances. For any (Control
Substance) products Import and Export *** subjected to your country
government laws /control substance ACT.
Note /Government Notification:
N/A
|
|










