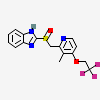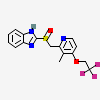
Lansaprazole Cas No. 103577-45-3
The active ingredient in Lansoprazole
Delayed-Release Capsules, Lansoprazole for Delayed-Release Oral
Suspension and Lansoprazole SoluTab Delayed- Release Orally
Disintegrating Tablets is lansoprazole, a substituted benzimidazole,
2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl] methyl] sulfinyl]
benzimidazole, a compound that inhibits gastric acid secretion. Its
empirical.
Lansoprazole is a white to brownish-white odorless crystalline powder
which melts with decomposition at approximately 166°C. Lansoprazole is
freely soluble in dimethylformamide; soluble in methanol; sparingly
soluble in ethanol; slightly soluble in ethyl acetate, dichloromethane
and acetonitrile; very slightly soluble in ether; and practically
insoluble in hexane and water.
Lansoprazole is stable when exposed to light for up to two months. The
rate of degradation of the compound in aqueous solution increases with
decreasing pH. The degradation half-life of the drug substance in
aqueous solution at 25°C is 18 hours at pH 7.0.
Lansoprazole is supplied in delayed-release capsules, in delayed-release
orally disintegrating tablets for oral administration and in a packet
for delayed-release oral suspension.
The delayed-release capsules are available in two dosage strengths: 15
mg and 30 mg of lansoprazole per capsule. Each delayed-release capsule
contains enteric-coated granules consisting of 15 mg or 30 mg of
lansoprazole (active ingredient) and the following inactive ingredients:
hydroxypropyl cellulose, low substituted hydroxypropyl cellulose,
colloidal silicon dioxide, magnesium carbonate, methacrylic acid
copolymer, starch, talc, sugar sphere, sucrose, polyethylene glycol,
polysorbate 80, and titanium dioxide. Lansoprazole SoluTab
Delayed-Release Orally Disintegrating Tablets are available in two
dosage strengths: 15 mg and 30 mg of lansoprazole per tablet. Each
delayed-release orally disintegrating tablet contains enteric-coated
microgranules consisting of 15 mg or 30 mg of lansoprazole (active
ingredient) and the following inactive ingredients: lactose monohydrate,
microcrystalline cellulose, magnesium carbonate, hydroxypropyl
cellulose, hypromellose, titanium dioxide, talc, mannitol, methacrylic
acid, polyacrylate, polyethylene glycol, glyceryl monostearate,
polysorbate 80, triethyl citrate, ferric oxide, citric acid,
crospovidone, aspartame **, artificial strawberry flavor and magnesium
stearate. Lansoprazole for Delayed-Release Oral Suspension are available
in two dosage strengths: 15 mg and 30 mg of lansoprazole per packet.
Each packet of delayed-release oral suspension contains enteric-coated
granules consisting of 15 or 30 mg of lansoprazole (active ingredient)
and the following inactive ingredients (inactive granules):
confectioner's sugar, mannitol, docusate sodium, crospovidone, citric
acid, sodium citrate, magnesium stearate, and artificial strawberry
flavor. The lansoprazole granules and inactive granules, present in unit
dose packets, are constituted with water to form a suspension and
consumed orally.
|
|